First off, I can tell you that if you are any kind of endurance athlete or a coach that trains endurance athletes NIRS is likely going to be worth your while. If you are a CrossFit athlete it is also probably going to provide you with useful information that will be helpful for more pointedly building up your aerobic system. Same goes for a field sport athlete where the ability to maintain repeated short burst sprint performance is the name of the game and this ability is primarily refueled by the aerobic system. BUT, if you are strength or hypertrophy athlete and are chasing increases in 1RMs and lean body mass – we know far less (isn’t that unfortunately almost always the case). The other setting not related to performance or athletics where NIRS may be applicable is in the clinic, in unhealthy sedentary individuals where NIRS could provide a numerical representation of mitochondrial function improvement.
WTF is NIRS or Near-Infrared Spectroscopy anyways?
Great question.
“Near-infrared light can penetrate biological tissues with less scattering and absorption than visible light and consequently offers advantages for imaging and quantitative measurements. In its simplest form, a NIRS device consists of a light-source emitting two or more wavelengths of light in the near-infrared range (650-1000 nm) into the tissue of interest and a detector placed at a known distance from the source(s). The chromophores haemoglobin (Hb) and myoglobin (Mb) are oxygen carriers in blood and skeletal myocytes respectively and their absorbance of near infrared light differs depending on whether they are in an oxygenated or deoxygenated state.”
-Jones et al. 2016
What does NIRS measure?
“NIRS measurements reflect the balance of O2 delivery to working muscles and muscle O2 consumption in capillary beds”
-Ufland et al. 2012
“Combining NIRS with simple physiological interventions, such as venous or arterial occlusions, allows quantitative measurements to be made from skeletal muscle. This provides a tool for assessing two major determinants of the capacity of muscles to exercise: O2 delivery and O2 utilization. The non-invasive nature of NIRS makes it an appealing technique for use in a dynamic environment and for activities of daily living.”
-Jones et al. 2016
As lifters, WHY do we care?
On a metabolic level, if we are after producing insane amounts of work output, what we are after is the ability to dissipate heat and acid, as well as restore phosphocreatine as efficiently and quickly as possible. NIRS may in fact be a viable proxy to the latter, which has enormous potentially. NIRS is also going to show you if you are occluding and therefore not able to get waste products out of the muscle in question, again potentially huge for sports that involve chasing work capacity on repeated efforts or even hypertrophy, but probably not as huge for sprint and powerlifting athletes.
Below are a bunch of graphs from Ufland et al. 2014. What the researchers did was have the subjects plantar flex their foot continuously for 30 sec to voluntary muscular failure. You can see that in the first gray bar in all the graphs. Then they waited, 10, 30, 60, 120, and 300 seconds before running another 10 seconds of planter flexion with force production measurements. You can see it took somewhere between 3 to 5 minutes for muscle oxygenation to come back to baseline levels.
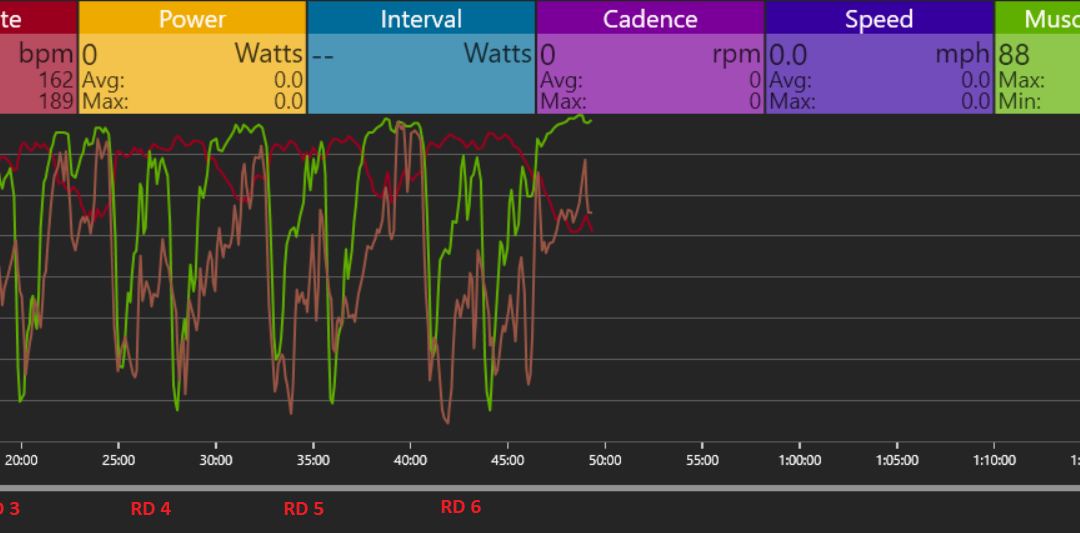
Now let’s take a look at my NIRS data (monitor is on my Right Rectus Femoris) from 6 Rounds of the 20/40 protocol of MASS 2. What I recommend whenever you get into a new technology is to just watch for months, if not years before you start changing everything based on the data. Be on the front edge, but be skeptical.
There are tried and true principals in this field and most people don’t need complicated. They need to get better at simple shit and loading it up. They have to be consistent over a long period of time to accumulate enough work and volume to get actually get stronger and change body composition. They also have to be able to recover from the above work. That said, new age tools are going to play a role in humans doing things no one thought was possible. This doesn’t mean that Marge needs NIRS measurements on her leg while she is gardening or even learning how to goblet squat (although using it as clinical measure of mitochondrial function could be helpful). But, other humans who are chasing the 1% of the 1% may benefit…that is once we figure out what the hell all this data means.
In the graph above, I lifted a little over 61,000 pounds in 46 min.
Each round was:
10 TB DLs (Low Bar Position)
10 Bench Presses
10 Rows
10 Back Squats
- B Incline Bench
With 3 minutes rest between each round.
*As would be expected, all of these were in the 50-60% 1RM range
The first drop in the above graph in every round is the TB DL (if this is on the high handles you won’t even really get a reduction in muscle O2, thus don’t be a puborectalis. If you have the biomechanics, flip the bar instead of doing rack pulls from your knees). You can see that I recoup muscle oxygen in the legs (but not fully) during the subsequent bench and the row. I then knock muscle O2 down almost to zero on the Back Squat. After this, I have essentially 4 minutes with my legs doing next to nothing. I sit in a chair with my legs extended and you can see at the start of every round I fully come back to baseline muscle oxygen levels even though my heart rate is progressively unable to come down in that time frame and by round 5 my mind is completely blank and all I can think of is answering the bell.
Thus, overall central fatigue seems to be playing a larger role than the legs not being able to recover in the current protocol (cerebral NIRS measurements would likely support this). Furthermore, my guess is that if Coach Davis was running this protocol based off real time data, I would likely get way less rest in the beginning rounds and more in the latter and I would go until I could either no longer desaturate or fully recover O2. Sounds like weight room S&M to me and if he made me do a 7th round I might attempt to kick him in the shins, but I would be down in the name of science. Now will that change in protocol based on NIRS results in better gains, more volume overall, less injury risk? Well…
We don’t know…
Aaron Davis and his colleagues/mentors are probably the most experienced utilizing this technology in the “underground” S&C world. From the eval side, what they are utilizing it for is to look for limiters…Can the athlete actually desaturate muscle O2? Can they occlude and at what percentages of 1RM? Can they restore muscle oxygenation and how fast? Does breathing affect this? Is their heart the limiter or is it peripheral capillarization? Once identified these are all aspects that can be trained and then remeasured.
Yet, the thing that most interests me is an auto-regulatory hypertrophy protocol based off NIRS data in real-time. We have zero published longitudinal studies that I am aware of on this topic and the possibilities will get you torqued if you like graphs and individualized lifting protocols. We know from the research that some athletes adapt to only 2 sets and some need 8 (this volume issue has been covered more thoroughly here.) If NIRS is a way to figure this out on a daily basis. Shit! My mind is already spinning with possible study designs and Coach Davis and I have a 12ish week cross-over design in the works for 2018.
But, what do we know?
Muscle desaturation during squatting seems to be similar to other endurance modalities of training.
“The extent of muscle deoxygenation during resistance exercise appears similar to values previously reported in speed skaters skating in a low position, alpine skiers during a simulated giant slalom event, and sprinters performing the Wingate anaerobic power test.”
-Hoffman et al. 2003
Longer periods of muscle desaturation results in larger spikes in an exercise stimulated growth hormones response, whether this would have any impact on long-term hypertrophy results is highly debatable.
“The results attained in this study confirm previous investigations that have demonstrated that growth hormone, but not testosterone, concentrations are influenced by tissue ischemia in actively contracting muscle.”
-Hoffman et al. 2003
Moderate and high loads result in similar muscle O2 desaturation, but moderate load training takes significantly longer to come back to baseline.
“Muscle damage that occurs as a result of heavy resistance exercise is thought to be requisite for muscle hypertrophy and increases in strength. Thus, it would appear that resistance-training programs designed to invoke a greater extent and duration of muscle ischemia would result in increased muscle hypertrophy…Despite similar deoxygenation levels during each training session, there was a 44.1% longer delay in the start of reoxygenation after LI compared with HI. The phenomenon of delayed reoxygenation, as a function of intensity and volume during resistance exercise, has not been previously seen. It is likely that the higher lactate accumulation seen during LI facilitated a greater O2Hb dissociation (Bohr effect). In addition, previous research has also demonstrated that O2Mb dissociation will follow a similar pattern of O2Hb dissociation when exercise intensity is maximal. Considering that subjects were exercising at intensities that approximated their RM for each exercise intensity, it appears that the duration of exercise performed at a maximal effort may be more important than the relative intensity of exercise in affecting muscle oxygen recovery kinetics.”
-Hoffman et al 2003
Performing moderate or high intensity exercise under hypoxic conditions results in more metabolic stress than under normal oxygen levels, whether this would result in increased adaptive changes in strength or hypertrophy in longer term trials is currently unknown.
“The main findings of this study demonstrate that moderate load resistance exercise performed in hypoxia can enhance markers of metabolic stress and muscle activation in well-trained participants.”
-Scott et al 2017
NIRS is an applicable way to determine maximal steady state running speed (as opposed to lactate threshold testing).
“NIRS was used to predict maximal steady state running speed in 12 distance trained runners and triathletes. Percent muscle oxygen saturation breakpoint, as determined by NIRS during an incremental exercise test, predicted maximal steady state running speed as well as the traditional blood lactate concentration methods currently used in this group of aerobically trained athletes. The ability of percent O2 saturation to predict MSS in anaerobically trained athletes and in heterogeneous groups of subjects is yet to be determined.”
-Synder et al. 2009
NIRS is more accurate than heart rate for monitoring intensity in long distance events with varying difficulties such as trail running and mountain cycle racing.
“Whereas HR and even V02 respond with a time delay to the onset of exercise, tissue saturation index (TSI) provides much quicker feedback. Unlike HR, which is influenced by thermoregulatory disturbance, dehydration, and mental competition stress, the TSI remains unaffected by these disturbances. Especially when exercising with continuously changing intensity (eg, running in hilly terrain). Thus, the wireless and lightweight NIRS devices could provide an alternative method to monitor exercise intensity.”
-Born et al. 2017
NIRS is a noninvasive and applicable way to measure mitochondrial function.
“The recovery of mVO2 after exercise was fit to a monoexponential curve, and a rate constant was calculated (directly related to mitochondrial function)…NIRS measurements of mitochondrial function can be made with both voluntary and electrically stimulated exercise contractions.”
Ryan et al. 2012
Finally, both aerobic and resistance training modalities improve mitochondrial function.
“A linear increase in mitochondrial capacity was found with a group average of 64 ± 37% improvement after four weeks of exercise training (p < 0.05). Mitochondrial capacity declined exponentially upon cessation of exercise training, with a mean half-time of ~7.7 days.”
– Ryan et al. 2013“We determined the effects of chronic resistance exercise training (12 weeks) on skeletal muscle mitochondrial function. We show that resistance exercise training elicits both quantitative and qualitative adaptations in skeletal muscle mitochondrial respiration. This data suggest that resistance exercise training is a means of augmenting mitochondrial respiratory capacity and function in skeletal muscle.”
-Porter et al. 2015
Other research quotes you may find interesting.
“Magnetic resonance spectroscopy (MRS) has the ability to measure active forms of high-energy phosphorus metabolites and intramuscular pH in vivo. With MRS, mitochondrial capacity can be assessed by the kinetics of phosphocreatine (PCr) resynthesis after exercise. However, MRS is costly and has limited availability…The advantage of the NIRS approach to measuring muscle mitochondrial function is the lower cost and wider spread availability”
The principal finding of this study was that endurance trained athletes had approximately twice the mitochondrial capacity of inactive controls as measured with NIRS. Mitochondrial capacity was measured as the recovery rate of mVO2 and assumed to be inversely related to the time constant, analogous to the recovery of PCr using MRS. Assessments of oxidative capacity using the rate of PCr recovery measured with 31P-MRS has also produced similar results. Both track athletes and competitive rowers have been observed to have PCr recovery rates approximately twice as fast as untrained controls in their respective active muscle tissue.”
-Brizendine et al 2013
“Both the number of mitochondria and the function of mitochondria are related to exercise performance. Reduced mitochondrial function and/or density is also associated with several pathological conditions, including diabetes, aging, and neuromuscular disease. Because of the importance of mitochondrial function on both health and physical performance, the development of novel, cost-effective methodologies to study mitochondrial health is critical.
The most widely used 31P-MRS assessment for mitochondrial function is the recovery rate of phosphocreatine (PCr) following exercise. Using the assumption of equilibrium for the creatine kinase reaction, the recovery rate of PCr after exercise is a function of mitochondrial ATP production, which has been validated against in vitro measurements of enzyme activity and high-resolution respirometry.
This [alternative] NIRS approach has been shown to be reproducible and independent of exercise intensity, detects the expected differences between untrained and trained individuals, as well as paralyzed and nonparalyzed individuals, and can track changes in mitochondrial function with exercise training and detraining. In the present study, we cross-validated NIRS measurements of skeletal muscle oxidative capacity with 31P-MRS measurements of skeletal muscle oxidative capacity in a population of young healthy adults.
This study found that NIRS-measured recovery kinetics of mVO2 were not statistically different from and correlated well with 31P-MRS-measured recovery kinetics of PCr after shortduration exercise. Furthermore, both NIRS and 31P-MRS measurements exhibited excellent reproducibility in the current experimental conditions.”
Ryan et al. 2013
“More importantly, PCr recovery is also thought to be oxygen dependent. For example, data on isolated canine gastrocnemius muscle have suggested that O2 availability was a major contributor to PCr recovery: the recovery of contracting skeletal muscle could only be observed with O2 reperfusion after a short period of complete ischaemia.
NIRS measurements reflect the balance of O2 delivery to working muscles and muscle O2 consumption in capillary beds. NIRS is well suited to the measurement of muscle oxygenation during exercise, as well as during recovery periods
In the present study, we observed significant decreases in muscle force production capacity following a 30-s MVC. After 10 s for example, mean Torque was decreased by 13% (Fig. 1). This is consistent with the result reported after a 30-s cycling sprint, where MVC decreased by approximately 15% in the early recovery phase. After 30 s, muscle force capacity recovered in a somewhat linear fashion to be complete within 300 s in the occlusion free condition.
Finally, the present recovery time was also comparable to that of PCr measured after a 30-s plantar-flexion exercise, which elicited a maximal muscle activation (Walter et al., 1997). Taken together, these data are therefore in line with the strong association generally described between muscle force capacity and PCr recovery
Present results confirm the role of muscle oxygenation in muscular force recovery during repeated-maximal efforts in man. However, the importance of postexercise muscle reoxygenation for force recovery is only moderate. These findings have important implications for the understanding of the mechanisms responsible for fatigue during repeated-maximal efforts, as well as for the development of optimized training interventions.”
-Ufland et al. 2014
“While neuromuscular performance (i.e., maximal sprinting speed) remains the strongest determinant of repeated sprint performance, present findings lend indirect support to the implementation of different forms of training programs aimed at targeting muscle metabolic recovery between sprints (e.g., submaximal endurance-based or high-intensity interval training). Optimal training programs should therefore include a well-balanced mixture of both strength/speed and conditioning work, with the proportion of both to be selected based on the athletes’ profile, specific sport demands and training phases over the competitive year. Finally, present results also highlight the promising potential of the present NIRS-derived method to assess m VO2. There is no doubt that the use of such a non-invasive, handy and quick (4 min are required to determine m VO2 ) method will increase in the field in the future; this will likely open new perspectives for both athletes screening and the assessment of training effects in athletes.”
-Ufland et al. 2012
REFERENCES for you to peruse.
- Ahmadi S, Sinclair PJ, Foroughi N, Davis GM. Monitoring muscle oxygenation after eccentric exercise-induced muscle damage using near-infrared spectroscopy. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2008;33(4):743-752.
- Austin KG, Daigle KA, Patterson P, Cowman J, Chelland S, Haymes EM. Reliability of near-infrared spectroscopy for determining muscle oxygen saturation during exercise. Research quarterly for exercise and sport. 2005;76(4):440-449.
- Bae SY, Hamaoka T, Katsumura T, Shiga T, Ohno H, Haga S. Comparison of muscle oxygen consumption measured by near infrared continuous wave spectroscopy during supramaximal and intermittent pedalling exercise. Int J Sports Med. 2000;21(3):168-174.
- Binzoni T, Cooper CE, Wittekind AL, et al. A new method to measure local oxygen consumption in human skeletal muscle during dynamic exercise using near-infrared spectroscopy. Physiol Meas. 2010;31(9):1257-1269.
- Binzoni T, Cooper CE, Wittekind AL, et al. A new method to measure local oxygen consumption in human skeletal muscle during dynamic exercise using near-infrared spectroscopy. Physiol Meas. 2010;31(9):1257-1269.
- Born DP, Stoggl T, Swaren M, Bjorklund G. Near-Infrared Spectroscopy: More Accurate Than Heart Rate for Monitoring Intensity in Running in Hilly Terrain. Int J Sports Physiol Perform. 2017;12(4):440-447.
- Brizendine JT, Ryan TE, Larson RD, McCully KK. Skeletal muscle metabolism in endurance athletes with near-infrared spectroscopy. Med Sci Sports Exerc. 2013;45(5):869-875.
- Buchheit M, Abbiss CR, Peiffer JJ, Laursen PB. Performance and physiological responses during a sprint interval training session: relationships with muscle oxygenation and pulmonary oxygen uptake kinetics. European journal of applied physiology. 2012;112(2):767-779.
- Buchheit M, Cormie P, Abbiss CR, Ahmaidi S, Nosaka KK, Laursen PB. Muscle deoxygenation during repeated sprint running: Effect of active vs. passive recovery. Int J Sports Med. 2009;30(6):418-425.
- Buchheit M, Simpson BM, Peltola E, Mendez-Villanueva A. Assessing maximal sprinting speed in highly trained young soccer players. Int J Sports Physiol Perform. 2012;7(1):76-78.
- Buchheit M, Ufland P, Haydar B, Laursen PB, Ahmaidi S. Reproducibility and sensitivity of muscle reoxygenation and oxygen uptake recovery kinetics following running exercise in the field. Clin Physiol Funct Imaging. 2011;31(5):337-346.
- Crum EM, O’Connor WJ, Van Loo L, Valckx M, Stannard SR. Validity and reliability of the Moxy oxygen monitor during incremental cycling exercise. European journal of sport science. 2017;17(8):1037-1043.
- Ferrari M, Muthalib M, Quaresima V. The use of near-infrared spectroscopy in understanding skeletal muscle physiology: recent developments. Philos Trans A Math Phys Eng Sci. 2011;369(1955):4577-4590.
- Fiogbe E, de Vassimon-Barroso V, de Medeiros Takahashi AC. Exercise training in older adults, what effects on muscle oxygenation? A systematic review. Arch Gerontol Geriatr. 2017;71:89-98.
- Goto M, Nirengi S, Kurosawa Y, Nagano A, Hamaoka T. Effects of the Drop-set and Reverse Drop-set Methods on the Muscle Activity and Intramuscular Oxygenation of the Triceps Brachii among Trained and Untrained Individuals. J Sports Sci Med. 2016;15(4):562-568.
- Grassi B, Quaresima V. Near-infrared spectroscopy and skeletal muscle oxidative function in vivo in health and disease: a review from an exercise physiology perspective. J Biomed Opt. 2016;21(9):091313.
- Habazettl H, Athanasopoulos D, Kuebler WM, et al. Near-infrared spectroscopy and indocyanine green derived blood flow index for noninvasive measurement of muscle perfusion during exercise. Journal of applied physiology. 2010;108(4):962-967.
- Hamaoka T, Katsumura T, Murase N, et al. Muscle oxygen consumption at onset of exercise by near infrared spectroscopy in humans. Advances in experimental medicine and biology. 2003;530:475-483.
- Hoffman JR, Im J, Rundell KW, et al. Effect of muscle oxygenation during resistance exercise on anabolic hormone response. Med Sci Sports Exerc. 2003;35(11):1929-1934.
- Ihsan M, Abbiss CR, Lipski M, Buchheit M, Watson G. Muscle oxygenation and blood volume reliability during continuous and intermittent running. Int J Sports Med. 2013;34(7):637-645.
- Jan Kodejška MM, Jiri Balas. Forearm muscle oxygenation during sustained isometric contractions in rock climbers. Acta Universatatis Carolnae Kinathropologia. 2015;51:48-55.
- Jian-Guo Bau Y-FC, Hua-Jian Lin. Effects of Low-Intensity Exercise Training on Tissue Oxygen Saturation of Lower-Extremity in Community-Dwelling Older Adults. 7th WACBE World Congress on Bioengineering. 2015:130-133.
- Jones S, Chiesa ST, Chaturvedi N, Hughes AD. Recent developments in near-infrared spectroscopy (NIRS) for the assessment of local skeletal muscle microvascular function and capacity to utilise oxygen. Artery Res. 2016;16:25-33.
- Kime R, Im J, Moser D, Nioka S, Katsumura T, Chance B. Noninvasive determination of exercise-induced vasodilation during bicycle exercise using near infrared spectroscopy. Med Sci Monit. 2009;15(3):CR89-94.
- Koga S, Barstow TJ, Okushima D, et al. Validation of a high-power, time-resolved, near-infrared spectroscopy system for measurement of superficial and deep muscle deoxygenation during exercise. Journal of applied physiology. 2015;118(11):1435-1442.
- Ladewig M, Robertson R, Nemoto EM. Muscle oxygenation by near infrared spectroscopy and lactate thresholds in endurance trained and recreationally active cyclists. Advances in experimental medicine and biology. 2003;510:273-278.
- Lanza IR, Nair KS. Mitochondrial metabolic function assessed in vivo and in vitro. Curr Opin Clin Nutr Metab Care. 2010;13(5):511-517.
- Leung TS, Wittekind A, Binzoni T, Beneke R, Cooper CE, Elwell CE. Muscle oxygen saturation measured using “cyclic NIR signals” during exercise. Advances in experimental medicine and biology. 2010;662:183-189.
- Marin T, Moore J. Understanding near-infrared spectroscopy. Adv Neonatal Care. 2011;11(6):382-388.
- Martin DS, Levett DZ, Mythen M, Grocott MP, Caudwell Xtreme Everest Research G. Changes in skeletal muscle oxygenation during exercise measured by near-infrared spectroscopy on ascent to altitude. Crit Care. 2009;13 Suppl 5:S7.
- Miyamoto N, Wakahara T, Ema R, Kawakami Y. Non-uniform muscle oxygenation despite uniform neuromuscular activity within the vastus lateralis during fatiguing heavy resistance exercise. Clin Physiol Funct Imaging. 2013;33(6):463-469.
- Mohler ER, 3rd, Lech G, Supple GE, Wang H, Chance B. Impaired exercise-induced blood volume in type 2 diabetes with or without peripheral arterial disease measured by continuous-wave near-infrared spectroscopy. Diabetes Care. 2006;29(8):1856-1859.
- Monroe DC, Gist NH, Freese EC, O’Connor PJ, McCully KK, Dishman RK. Effects of Sprint Interval Cycling on Fatigue, Energy, and Cerebral Oxygenation. Med Sci Sports Exerc. 2016;48(4):615-624.
- Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect. 2015;4(1):R1-R15.
- Motobe M, Murase N, Osada T, et al. Noninvasive monitoring of deterioration in skeletal muscle function with forearm cast immobilization and the prevention of deterioration. Dyn Med. 2004;3(1):2.
- Neary JP. Application of near infrared spectroscopy to exercise sports science. Can J Appl Physiol. 2004;29(4):488-503.
- Nielsen HB, Boesen M, Secher NH. Near-infrared spectroscopy determined brain and muscle oxygenation during exercise with normal and resistive breathing. Acta Physiol Scand. 2001;171(1):63-70.
- Pereira MI, Gomes PS, Bhambhani YN. A brief review of the use of near infrared spectroscopy with particular interest in resistance exercise. Sports medicine. 2007;37(7):615-624.
- Porter C, Reidy PT, Bhattarai N, Sidossis LS, Rasmussen BB. Resistance Exercise Training Alters Mitochondrial Function in Human Skeletal Muscle. Med Sci Sports Exerc. 2015;47(9):1922-1931.
- Racinais S, Buchheit M, Girard O. Breakpoints in ventilation, cerebral and muscle oxygenation, and muscle activity during an incremental cycling exercise. Frontiers in physiology. 2014;5:142.
- Roberts LA, Muthalib M, Stanley J, et al. Effects of cold water immersion and active recovery on hemodynamics and recovery of muscle strength following resistance exercise. Am J Physiol Regul Integr Comp Physiol. 2015;309(4):R389-398.
- Ryan TE, Brizendine JT, McCully KK. A comparison of exercise type and intensity on the noninvasive assessment of skeletal muscle mitochondrial function using near-infrared spectroscopy. Journal of applied physiology. 2013;114(2):230-237.
- Ryan TE, Brizendine JT, McCully KK. A comparison of exercise type and intensity on the noninvasive assessment of skeletal muscle mitochondrial function using near-infrared spectroscopy. Journal of applied physiology. 2013;114(2):230-237.
- Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. Journal of applied physiology. 2012;113(2):175-183.
- Ryan TE, Erickson ML, Brizendine JT, Young HJ, McCully KK. Noninvasive evaluation of skeletal muscle mitochondrial capacity with near-infrared spectroscopy: correcting for blood volume changes. Journal of applied physiology. 2012;113(2):175-183.
- Ryan TE, Southern WM, Brizendine JT, McCully KK. Activity-induced changes in skeletal muscle metabolism measured with optical spectroscopy. Med Sci Sports Exerc. 2013;45(12):2346-2352.
- Ryan TE, Southern WM, Reynolds MA, McCully KK. A cross-validation of near-infrared spectroscopy measurements of skeletal muscle oxidative capacity with phosphorus magnetic resonance spectroscopy. Journal of applied physiology. 2013;115(12):1757-1766.
- Saitoh T, Ooue A, Kondo N, Niizeki K, Koga S. Active muscle oxygenation dynamics measured during high-intensity exercise by using two near-infrared spectroscopy methods. Advances in experimental medicine and biology. 2010;662:225-230.
- Scott BR, Duthie GM, Thornton HR, Dascombe BJ. Training Monitoring for Resistance Exercise: Theory and Applications. Sports medicine. 2016;46(5):687-698.
- Scott BR, Goods PS, Slattery KM. High-Intensity Exercise in Hypoxia: Is Increased Reliance on Anaerobic Metabolism Important? Frontiers in physiology. 2016;7:637.
- Scott BR, Peiffer JJ, Goods PSR. The Effects of Supplementary Low-Load Blood Flow Restriction Training on Morphological and Performance-Based Adaptations in Team Sport Athletes. J Strength Cond Res. 2017;31(8):2147-2154.
- Scott BR, Slattery KM, Dascombe BJ. Intermittent hypoxic resistance training: does it provide added benefit? Frontiers in physiology. 2014;5:397.
- Scott BR, Slattery KM, Dascombe BJ. Intermittent hypoxic resistance training: is metabolic stress the key moderator? Med Hypotheses. 2015;84(2):145-149.
- Scott BR, Slattery KM, Sculley DV, Lockhart C, Dascombe BJ. Acute Physiological Responses to Moderate-Load Resistance Exercise in Hypoxia. J Strength Cond Res. 2017;31(7):1973-1981.
- Scott BR, Slattery KM, Sculley DV, Smith SM, Peiffer JJ, Dascombe BJ. Acute physiological and perceptual responses to high-load resistance exercise in hypoxia. Clin Physiol Funct Imaging. 2017.
- Slyaz. The physiological effects of blood flow restricted muscle stimulation. 2015.
- Snyder AC, Parmenter MA. Using near-infrared spectroscopy to determine maximal steady state exercise intensity. J Strength Cond Res. 2009;23(6):1833-1840.
- Southern WM, Ryan TE, Reynolds MA, McCully K. Reproducibility of near-infrared spectroscopy measurements of oxidative function and postexercise recovery kinetics in the medial gastrocnemius muscle. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2014;39(5):521-529.
- Tsujii T, Komatsu K, Sakatani K. Acute effects of physical exercise on prefrontal cortex activity in older adults: a functional near-infrared spectroscopy study. Advances in experimental medicine and biology. 2013;765:293-298.
- Ufland P, Ahmaidi S, Buchheit M. Repeated-sprint performance, locomotor profile and muscle oxygen uptake recovery: effect of training background. Int J Sports Med. 2013;34(10):924-930.
- Ufland P, Lapole T, Ahmaidi S, Buchheit M. Muscle force recovery in relation to muscle oxygenation. Clin Physiol Funct Imaging. 2012;32(5):380-387.
- Zafeiridis A, Kounoupis A, Dipla K, et al. Oxygen Delivery and Muscle Deoxygenation during Continuous, Long- and Short-Interval Exercise. Int J Sports Med. 2015;36(11):872-880.
- Zhang Z, Wang B, Gong H, Xu G, Nioka S, Chance B. Comparisons of muscle oxygenation changes between arm and leg muscles during incremental rowing exercise with near-infrared spectroscopy. J Biomed Opt. 2010;15(1):017007.




Recent Comments